Ozone Water Treatment is around for 120 years. But what is ozone? How is it produced? What’s its history? How is it used in the water industry and beyond? We investigate all of this (and more) today!
This is part of my deep dive on water and wastewater treatment!
Table of contents
What do you know about ozone?
You may have heard of ozone for two reasons:
- The ozone layer depletion, which increases the UV radiation levels on Earth
- And this band that used to dance on a plane.
So what is ozone water treatment, and how is it used in the water industry?
What is Ozone?
Ozone at its roots is just another form of oxygen. The only difference to dioxygen is that it features 3 instead of 2 atoms of oxygen.
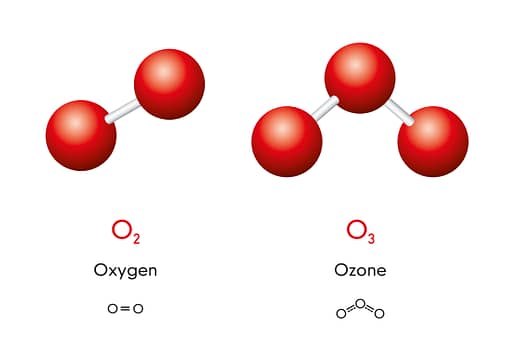
That’s fancy, sure, but it’s also much less stable. If you leave it alone, an ozone molecule will always return to its dioxygen form.
So if it’s so unstable, how can there be a natural ozone layer in the atmosphere?
How does an “ozone layer” happen in the atmosphere?
Well, if you bombard dioxygen with short-wave UV, you break it down to oxygen atoms that then recombine with dioxygen to form ozone.

That being said, we speak of an ozone layer, but in fact, ozone only represents about 0.002% of the oxygen up there, 10 to 50 kilometers above Earth. So it’s like saying that an Olympic swimming pool is an orange juice layer because your daughter dropped her orange juice in it.
But OK. 😄
How to create Ozone aside from UV radiation?
Next to UV radiation, there is another way to create ozone, which consists of striking dioxygen molecules with electrical discharges. And actually, that’s what some chemists have been doing through history, like Martinus Van Marum in 1785 – who discovered ozone without noticing – and Christian Friedrich Schönbein in 1839 – who finally noticed the discovery.

How did he do that? Well, while playing around with electricity and water in his laboratory, he noticed a strong smell that reminded him of what you may experience if you walk past a bolt of lightning.

He then recalled his greek lessons and the verb “ozein” which means “to smell,” and decided to call his new discovery ozone.
Understanding Ozone
It would take some more years to understand that ozone was actually tri-oxygen, but many hipsters at the end of the 19th century and even the beginning of the 20st praised this new gas for its “fresh” smell that HAD to have positive health effects.
Beaumont, in California, changed its city slogan to “Beaumont: Zone of Ozone” and naturalist Henry Henshaw wrote how he enjoyed an atmosphere with enough ozone to sustain the necessary energy to work.
The thing is that Professor Schönbein himself already reported how he experienced chest pains and irritation of its mucous membranes while working on ozone. It took some decades more, of testing it on frogs, birds and rabbits, but it finally appeared that ozone was IN FACT dangerous for human health.
Ozone is one of the strongest oxidants!
Why so? Simply because ozone is actually one of the most powerful oxidants on Earth. You wouldn’t drink bleach, would you?

Well, ozone’s oxidation potential is about 50% higher than chlorine.
Hey Henry, still want some energy to work?
Knowing this, ozone was tested in multiple applications. For instance, during the first world war, some hospitals in London tried ozone as a disinfectant for wounds.
Fact, it killed the bacteria. But fact as well, it had this little drawback to also kill skin and human tissues.
The birth of Ozone Water Treatment
But there’s one field of experimentation where ozone would do great, and that is, of course, water treatment.
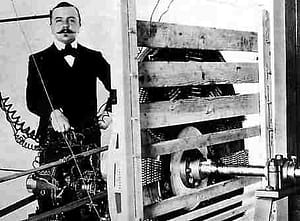
Towards the end of the 19th century, some of the rockstars of electrical engineering had developed the first ozone generators. Like Nikola Tesla, in the United States, that founded “Tesla Ozone Co.” or Werner von Siemens a bit before in Germany, that actually built the very first ozone generator.

Siemens did not stop there, he also wrote a book on the application of ozone in water, which ignited several pilot plants in Europe.

The first ever Ozone Water Treatment: Oudshoorn (NL) in 1893
In 1886, several studies had shown that ozonized air could sterilize polluted water, which led the city of Oudshoorn in the Netherlands to build the first-ever ozone treatment for drinking water production in 1893.
This demonstration plant gained a lot of attention, especially from French scientist Marius Paul Otto, who refined the concept to build and commission the ozone treatment plant of Nice in 1907.
One hundred fourteen years later, that plant – after of course several upgrades – is still running, making it the longest operating ozone water plant in the world.
Early growth of Ozone Water Treatment
The next decade would see about 50 ozone plants built in Europe, of which a good half in France.
The first world war brutally stopped this rapid expansion. But not for the reasons you’d spontaneously expect.
Indeed, a lot of chemical research occurred during this conflict, leading to a better understanding and mastering of chlorine, which happened to not only kill people, but also pretty well disinfect water.
A mighty competitor: Chlorine
The next fifty years would hence be the ones of chlorine – that would gain on the go the title of major public health achievement of the 20th century, while ozone treatment hardly doubled its installed base over the same period of time.
But a lot would change in the 70s, starting with J.J. Rook’s research on hypochlorous and hypobromous acids.

He indeed proved that those reacted with organic matter to create many water disinfection byproducts.
THMs, a major drawback of Chlorine
The most well-known are the four horsemen of the apocalypse: Chloroform, Bromodichloromethane, Dibromochloromethane, and Bromoform.
Pronouncing numbers 2 and 3 is everything but easy, so let’s simply call all of those trihalomethanes or even simpler THMs.
Some THMs are proven to be carcinogenic, while others are heavily suspected. So the market started looking for chlorine alternatives, especially when chemical precursors were present in the raw water.
The fear of THM revived ozone water treatment!
And look who’s still around? Exactly, ozone water treatments. But with a fresh look.
Indeed, we’ll have to go back a bit in time, to the 60s in Zürich, Switzerland.
New entrants in the ozone industry
At that time, engineers at Brown Boveri and Company were investigating the problem of line breakers in high tension wires. They wanted to find out how to prevent the electrical discharges that occur when these wires are separated.
Did they solve that problem? Honestly I don’t know.
But they swiftly noticed that their experiences were creating quite a lot of ozone.
Improving ozone generation through the corona discharge
Without directly realizing it, they heavily improved the corona discharge process to produce ozone, giving ozone generators their tubular shape most of them still have nowadays.
This enabled better energetical efficiencies, but also higher concentrations of ozone in the produced gas.
In 1975, BBC launched its ozone department, swiftly followed by Wedeco in Germany in 1976, two departments that now respectively belong to SUEZ and Xylem.
Those companies would enable to safely reach larger plant capacities, thus getting a scale effect that lowered the operating costs, compared with the early ozone pioneers.
Significant Ozone Water Treatment milestones
In 1978, the world’s largest ozone plant was built in Sipplingen, Germany, next to the Lake Constance. Although this record didn’t hold long, this plant is open for visits, and if you ever pass by the south-west of Germany, I’d recommend you to give it a chance, as they kept all the evolutions of the various ozone generators they used over time.

The next milestone for ozone treatment happened in 1985, when the city of Los Angeles built the new largest ozone plant in the world, with a capacity of 200kg of ozone per hour, and a brand new approach.
Ozone generation from Oxygen
Indeed, until then, ozone was produced, as we’ve seen, by breaking the dioxygen molecules present in dry air. But that meant, taping in only 21% of the carrier gas, with a lot of nitrogen, just flowing around for not much.
The Los Angeles plant changed this by using pure oxygen as a feed gas. Imagine, now you’re no longer using just 21% of your feed gas, but almost 100%.
As a consequence, ozone concentrations went up.
Towards higher ozone concentrations
While it was already solid performance from air, to reach 2 to 4 weight percent of ozone, the era of pure oxygen enabled to reach 8, 10, 12 and nowadays 16, 18 or even 20 weight percent ozone concentration in some applications.
Talking of applications, this new performance scale opened the door to many other uses of ozone, from sludge reduction to pulp & paper bleaching, through COD removal or ozonolysis – that last process having the slightly annoying tendency to explode.
Ozone Water Treatment today
Nowadays, ozone water treatments are a solid number two in disinfection applications, just behind chlorine, and a good solution in many other fields, like wastewater reuse or removal of micropollutants.
Now that’s already a lot for a 101 on ozone treatment, so I’ll stop here.
But stay tuned, because we’ll soon dive a bit deeper into the matter with Jim Lauria, VP of Mazzei Injectors, and a board member of the International Ozone Association.
If you like the 101 format, tell me in the comments or send me a direct message! I’m also very open to suggestions as per the next technologies we shall address or review.
See you Soon!
BONUS: Where to find ozone?
Oh, by the way, if you never smelled any ozone, you can try to open an electrical transformer door, don’t worry; you won’t die from such a short exposure to ozone, but the transformer itself might be quite a hazard. Another option is to leverage your next visit to the office, to smell the copy room. Quite often, if there’s no sufficient ventilation, you’ll find some ozone there.
Thank me later, bye!

